Natural Diamond
Boutique
Discover an ever-changing collection of timeless Diamond Jewellery that complements you and completes your look. Make the right choice by buying your favourite Diamond Jewellery from Madurai’s best Diamond jewellery shop. Traced all the way back to 1937 Appu Diamonds is known for its Quality, trust and wide range of collections.
FEATURED PRODUCTS
Check out our latest seasonal collections
Our core value
Diamonds are the Symbol of eternal love, purity, status and strength. We use Natural Certified Diamonds when crafting hand-made pieces to make you shine brighter. Our choice for diamond is the perfect one & Every piece crafted with them truly deserves something special from us!
We’re dedicated to bringing you the highest quality diamonds at the best prices on the internet, with the exquisite experience and personal service you’d expect buying from us.

ENGAGEMENT & WEDDING
Solitaires Ring Collections
GET YOUR CUSTOM DESIGN
DIAMOND JEWELLERY!
Have a design on your mind ? Dont know where to get the same design jewellery? You came to right place. Yes, If you wish to order a personalized or customized piece of jewellery to be made with gold, diamonds and gemstones, we are here for you.
- Any type of design inspiration or your own design can be made.
- Custom wordings or letters can also be added to existing designs.
- Old jewellery can be exchanged to make new custom jewellery.
- All custom designed Diamond jewellery comes with international certificates.
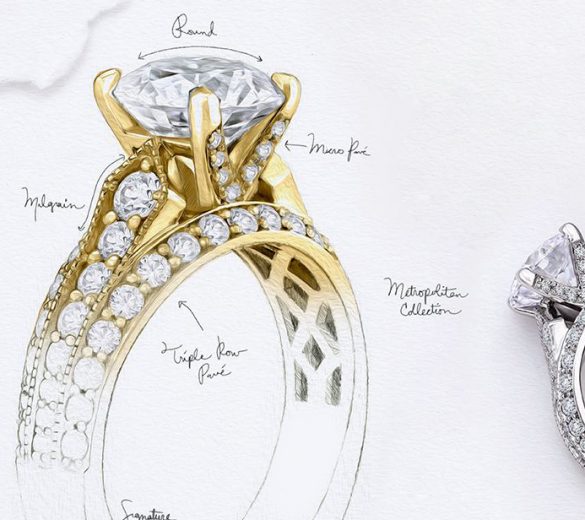
FEATURED PRODUCTS
-
 Diamond Stud
$229
Diamond Stud
$229
-
 Diamond Ring - Men
$229
Diamond Ring - Men
$229
-
 Diamond Ring - Women
$229
Diamond Ring - Women
$229
NEW PRODUCTS
-
 Diamond Stud
$229
Diamond Stud
$229
-
 Diamond Ring - Men
$229
Diamond Ring - Men
$229
-
 Diamond Ring - Women
$229
Diamond Ring - Women
$229
FOLLOW US ON INSTAGRAM
Follow us and stay connected with us!
Join us and get Special discounts every month
Subscribe to our Newsletter and get special offers regularly.
WHAT OUR CLIENTS SAY
Good place to buy jewellery. Cooperative and guides you to buy in a proper way. Download any designs from web, they convert to diamond ornaments. Highly Recommended !
A great place to buy high-quality certified diamonds from! The owner is himself certified in diamond grading, and takes personal care throughout you buying the jewelry, from choosing the jewelry design to delivering the jewelry. We are very happy with the service!
One of the finest place to buy authentic diamonds and other precious stones jewelry. myself and family have bought multiple times and a happy customer for years.
DIAMOND JEWELRY BLOG
Read our blogs to know more about diamond selection, cut and latest design trends.
KNOW ABOUT DIAMOND CARAT WEIGHT
-
Posted by
admin
- 0 comments
HOW TO TAKE CARE OF YOUR DIAMOND?
-
Posted by
admin
- 0 comments
HOW TO BUY A DIAMOND
-
Posted by
admin
- 0 comments










 Yellow Gold
Yellow Gold
 White Gold
White Gold
 Rose Gold
Rose Gold
 Platinum
Platinum